Cytology is
the study of free cells from different organs of
the body. These cells may have been shed by the body itself, aspirated by tube
or needle; or scraped or washed from tissue surfaces. Therefore, effusions,
secretions, aspirates and scrapings are all used in diagnostic cytology.
Exfoliative cytology is the study of cells which are shed spontaneously from
epithelial surfaces of the body.
This spontaneous shedding is a function of normal epithelium. The epithelial surfaces undergo constant growth and so they continue to shed worn out cells which are replaced by new ones. However, malignant tumour cells exfoliate more readily than those from healthy tissues.
The detection of malignant cells in clinical specimens under microscopic examination is the most important role of diagnostic cytology. In addition, valuable information may be obtained about infections and infestations, reproductive defects and hormonal status.
Clinical specimens are obtained with little or no discomfort to the patient.
This spontaneous shedding is a function of normal epithelium. The epithelial surfaces undergo constant growth and so they continue to shed worn out cells which are replaced by new ones. However, malignant tumour cells exfoliate more readily than those from healthy tissues.
The detection of malignant cells in clinical specimens under microscopic examination is the most important role of diagnostic cytology. In addition, valuable information may be obtained about infections and infestations, reproductive defects and hormonal status.
Clinical specimens are obtained with little or no discomfort to the patient.
All clinical
specimens sent to the Cytology laboratory should be regarded as potentially
pathogenic. Therefore, all safety precautions applicable to Microbiology
laboratory should also be applied here. Safety cabinets are a must for
processing sputum. Disinfectant jars should be placed at strategic points on
the work bench and the staff should wear protective clothing.
Cytology has
become a strong diagnostic tool for the early detection and diagnosis of
malignancy in different organs of the body, in the assessment of hormonal
activity which is valuable in some cases of infertility and in the evaluation
of certain endocrine disorders.
In
exfoliative cytology, the microscopic evaluation is based upon observation and
characteristics of the basic cells. Since a majority of the cells are in the
form of single cast off cells lying in a fluid medium, there is no distortion
to their shapes. This is unlike histopathology where the arrangement of cell
aggregates is the basis of diagnosis of malignancy.
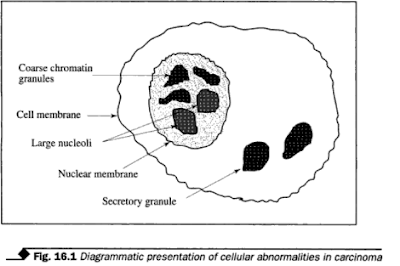
Most of the information from the study of discrete cells is based on the shape or size of the nuclei. Cytoplasm of the cells may only assist in identifying the cell type. Figure 16.1 shows some of the characteristic nuclear abnormalities associated with carcinomas. They are:
Most of the information from the study of discrete cells is based on the shape or size of the nuclei. Cytoplasm of the cells may only assist in identifying the cell type. Figure 16.1 shows some of the characteristic nuclear abnormalities associated with carcinomas. They are:
1. Nuclear
enlargement with no increase in the overall cell size, showing a decreased cytoplasm/nucleus
ratio.
2.
Irregularity of nuclear outline with variation in size and shape.
3.
Hyperchromasia. This is as a result of increased amounts of deoxyribonucleic
acid (DNA); the nuclei of malignant cells often stain more intensely with basic
dyes.
4. Multinucleate
due to abnormal cell division.
5. Uneven
distribution and variation in size of chromatin particles.
6. Increase
in size and number of nucleoli.
To achieve a meaningful result and interpretation, smears should be prepared from fresh specimens and fixed while still wet. The nature of the specimen, the age of cell population, method of collection and fixation will all affect the morphology of the cells. Most importantly, a prompt and proper fixation is essential.
FIXATION
A good
cytological fixative should penetrate rapidly with no distortion to the cell
morphology.
The
clinician, in most cases, prepares and fixes the smear in the clinic or at bed
side. The laboratory should make available to the wards and outpatients'
clinic, a container and carrier for smear fixatives in the form of a polythene
screw-capped coplin jar grooved to take up to 8-10 slides.
If the
smears are to be sent by post to a distant laboratory, aerosol fixative sprays
give an effective form of fixation. These aerosol sprays consist usually of an
alcohol and wax-the alcohol fixes
the smear
and the wax sets to provide a water-soluble protective film. Some laboratories
coat prefixed smears with glycerine or water soluble wax. Alternatively, the
smears may be air-dried after complete fixation and then placed in suitable
cardboard, plastic or wooden slide box-carrier designed and grooved to hold a
few slides.
Fluids of
larger volumes, e.g., pleural or peritoneal effusion, or sputum, should be sent
to the laboratory immediately after collection and smears can then be made.
The most
widely used fixatives are:
1.
Alcohol-ether (equal parts of diethyl ether and 95% alcohol) mixture.
2. 95%
alcohol with or without 3% glacial acetic acid.
3. A mixture
of seven parts of tertiary butyl alcohol and three parts of 95% alcohol.
4. Schaudinn's solution:
Saturated
aqueous mercuric chloride 66 ml
Absolute
ethyl alcohol 33 ml
Glacial
acetic acid 0.3 ml
Fixation
time in any of these fixatives is at least 15 minutes and maximum 7 days.
SUBDIVISIONS OF CYTOLOGY
For
practical purposes, cytology is subdivided as follows:
1. Gynaecological Cytology
(Gynae-cytology)
This deals with the cytology of the vagina, cervix and endometrium.
2. Non-gynaecological cytology This is the cytology of cells
suspended in body fluids.
3. Fine needle aspiration cytology
(FNAC) The
study of
cells aspirated from organs and structures of the body.
GYNAECOLOGICAL CYTOLOGY
There are
three most important applications of gynae-cytology:
(i) The
detection of malignancy--mainly cancer of cervix.
(ii)
Assessment of hormonal activity
(iii)
Identification of vaginal infection andinflammatory conditions.
Specimen Collection and Preparation of Smears
1. Cervical
smears Cervical smears are made from material collected with the help of a
speculum (a metal or plastic device) which is inserted into the vagina and
allows the uterine cervix to be readily visible. A specialised spatula known as
the Ayre spatula or cervical spatula (Fig. 16.2.) is used for collection. The
collection is made at the junction of the columnar epithelium by visualising
the cervix,
the spatula is inserted via the speculum into the cervical os and rotated
through 360 degrees. The material collected is quickly smeared over a
pre-labelled microscope slide and fixed immediately
2. Aspiration from the posterior
fornix With the aid
of a speculum, cellular material is collected from the posterior fornix, using
a disposable plastic pipette with a suction bulb (Fig. 16.3). Following
aspiration, smears are prepared and fixed immediately.
3. Vaginal smears Vaginal smears are valuable for the
assessment of hormonal function. Cellular material is collected by scraping the
upper third of the lateral wall of the vagina with a wooden spatula. The cells
are evenly and thinly smeared over a clean pre-labelled microscope slide and
fixed.
4. Endocervical smears This is used mainly for follow up
cases where a surgical treatment has been used after a cone biopsy has been
taken for assessment of dysplasia and malignancy or as a curative procedure. A
cotton tip swab is inserted into the endocervix and rotated gently to cover a
wide area of the endocervix. The material collected is smeared on a clean
pre-labelled microscope slide and fixed.
5. Endometrial aspiration This procedure has to be performed
under strict aseptic conditions so as not to introduce infection into the
patient. A cannula is inserted into the uterine cavity and the cellular
material is aspirated using a syringe. Thin smears are made on clean
pre-labelled slides and fixed.
STAINING
The most
commonly used stain in the gynae-cytology is the Papanicoloau stain. It is a
stain specially suitable for cervical smears. It gives sharp nuclear staining
and good differential colouring of acidophilic and basophilic cells. It makes
the cytoplasm transparent. There are other stains that can be effectively used
for diagnostic purposes in the gynae-cytology.
Papanicoloau Staining Method
This
staining method can be performed either manually when there are only a few
slides to deal with, or by using automatic staining machine when a large number
of slides is to be stained.
Solutions
1. Harris
haematoxylin (see Chapter 7)
2. Orange G
solution (OG 6) 0.5% Orange G (CI No.16230) in 95% alcohol 100 ml
Phosphotungstic
acid 0.015 g
3. Eosin
Azure 50 (E.A. 50 or E A 36):
0.5% light
green SF (Yellowish) (CI No. 42095) in 95% alcohol 45 ml
0.5% Bismark
brown Y (CI No.21000) in 95% alcohol 10 ml
0.5% Eosin
(C1 No.45380) in 95% alcohol 45 ml
Phosphotungstic
acid 0.2g
Saturated
aqueous lithium carbonat e1 drop
Mix well and
store in tightly capped, brown bottles.
Procedure for manual staining
1. Remove
smears from fixative.
2. Rinse in
descending grades of alcohol (80,70 and 50%) and water for 10 seconds each.
3. Stain in
Harris haematoxylin for 2 minutes.
4. Rinse in
water for 1-2 minutes.
5.
Differentiate in 1% acid alcohol until only the nuclei retain the stain (a few
seconds)
6. Wash and
blue in tap water for 3-5 minutes.
7. Transfer
to 70% alcohol for a few seconds.
8. Transfer
to 95% alcohol for a few seconds.
9. Stain in
OG 6 for 2 minutes.
10. Rinse in
2 changes of 95% alcohol.
11. Stain in
EA 50 for 2-4 minutes.
12. Rinse in
2 changes of 95% alcohol.
13.
Dehydrate in absolute alcohol, clear in xylene and mount in neutral synthetic medium.
Results
Nuclei:
|
Blue
|
Acidophilic (superficial) cells
|
reddish-pink
|
Basophilic (intermediate and parabasal
cells)
|
:blue/green
|
Candida albicans
|
Red to pale pink
|
Trichomonas vaginalis
|
grey green
|
Note E A 50 or E A 36 and OG 6 are
available commercially
Procedure for automatic staining Transfer fixed smears to:
Procedure for automatic staining
Transfer fixed smears to:
|
||
Trough
|
Solutions
|
Time in minutes
|
1
|
70% alcohol
|
1
|
2
|
50% alcohol
|
1
|
3
|
Distilled water
|
1
|
4
|
Harris haematoxylin
|
3
|
5
|
Distilled water
|
1
|
6
|
Tap water 0.5% HCI in
|
1
|
7
|
70% ethanol 1/2
|
1/2
|
8
|
Tap water
|
1
|
9
|
Tap water
|
1
|
10
|
70% alcohol
|
1
|
11
|
70% alcohol
|
1
|
12
|
95% alcohol
|
1
|
13
|
95% alcohol
|
1
|
14
|
OG 6
|
2
|
15
|
95% alcohol
|
1/2
|
16
|
95% alcohol
|
1/2
|
17
|
E A 50 or EA 36
|
3
|
18
|
95% alcohol
|
1
|
19
|
95% alcohol
|
1
|
20
|
Absolute alcohol
|
2
|
21
|
Absolute alcohol
|
2
|
22
|
Xylene
|
1
|
23
|
Xylene
|
2
|
Remove slides from the machine into
xylene dish and mount in a neutral synthetic resin medium.
|
||
Results Same as for manual technique.
HORMONE ASSESSMENT
As part of a
general investigation into the cause of human reproductive disorder and
sterility among women, hormone assessment is done.
Hormonal
activity can be evaluated on the basis of microscopic examination of
Papanicoloau stained vaginal smears. The karyopyknotic index (KPI) and the
maturation index (MI) are the two methods commonly used to assess hormone
activity.
Superficial
cornified squamous epithelial cells show condensed, deeply stained,
structureless (pyknotic) nuclei, with pink to red stained cytoplasm. The
calculation of KPI is done by counting a total of 200 squamous cells in a
Papanicoloau stained smear. The cornified squamous cells are expressed as a
percentage of the total number of assessment of the hormonal (oestrogenic) influence, smears are taken at about 3 or 4 days
interval throughout the menstrual cycle
The
maturation index (MI) is based on cell maturation which is determined by means
of the morphology and staining reactions of the noncornified squamous
epithelial cells. These are classified as superficial intermediate and
parabasal cells. A total of at least 200 squamous cells are counted and each
class of cells is expressed as a percentage. High oestrogenic activity is
indicated by a preponderance of superficial intermediate cells while low
activity is indicated by a predominance of parabasal cells. Another good
staining
method used
for the assessment of hormonal function is the Shorr's method.
Shorr's Staining Method
This method
uses a single differential staining solution. It gives less cytoplasmic
transparency and poorer nuclear definition than the Papanicoloau. It is not
very useful in the diagnosis of malignant cells.
Staining solution
Staining solution
|
|
50% ethyl alcohol
|
100 ml
|
Biebrich scarlet (CI No.26905)
Water-soluble
|
0.5 g
|
Orange G (CI No.16230)
|
0.25 g
|
Fast green FCF (CI No.42053)
|
0.075 g
|
Phosphomolybdic acid
|
0.5 g
|
Glacial acetic acid
|
1 ml
|
Procedure
1. Fix smear
in alcohol-ether for 1-2 minutes
2. Stain in
Shorr stain for 1-2 minutes.
3. Rinse in
70% alcohol to remove excess stain.
4. Transfer
to 95% alcohol for 3 seconds.
5. Transfer
to absolute alcohol for 3 seconds. 6. Clear in xylene and mount in a neutral
synthetic resin.
Results
Nuclei
|
Red
|
Superficial cornified cells :
|
Bright orange-red
|
Non-cornified cells :
|
Green-blue
|
There are
other stains which, though less effective, can be used in gynae-cytology. Some
of these are:
(i)
Haematoxylin and Eosin
(ii)
Acridine orange fluorescence technique
(iii)
Feulgen's reaction for DNA for researchwork.
Sex Chromatin (Barr bodies)
Sex
chromatin is present in about 30% of cells from the female whereas cells from
the male do not have the sex chromatin. The sex chromatin appears as a darkly
staining dot in the nucleus. It is present
in the cells
of the skin, cells of the buccal mucosa and in the cells of blood, notably the
leucocytes. Specimens are conveniently obtained by scrapings of the buccal
mucosa.
The Barr
bodies are normally seen attached to the nuclear membrane of the epithelial
cells. It is sometimes used to determine the sex of an individual.
Cresyl Fast Violet Acetate Method for Demonstrating Sex Chromatin
This is a
rapid, simple and effective method for demonstrating sex chromatin. It requires
no differentiation.
Staining solution
Cresyl fast
violet acetate 1.0 g
Distilled
water100 ml
Procedure
1. Fix smear
while still wet in 95% alcohol for three minutes.
2. Transfer
to 50% alcohol for a few seconds and then to distilled water.
3. Stain
with cresyl fast violet acetate solution for five minutes.
4. Rinse
quickly in tap water.
5. Dehydrate
with 95% alcohol, then with absolute alcohol.
6. Clear in
two changes of xylene and mount in a neutral synthetic resin medium.
Result
Sex
chromatin deeply stained
Cytoplasm faintly
stained.
Note
This method
is treated under Gynae-cytology for convenience. Specimens of bronchial
washings or lavage should be centrifuged and smears made and fixed immediately.
NON-GYNAECOLOGICAL CYTOLOGY
This aspect
of cytology involves the study of cells suspended in body fluids. The specimens
are varied and are taken from various parts of the body.
Sputum
Sputum
specimen is valuable for the study of respiratory tract disorders. It is used
in the diagnosis of the following abnormal conditions.
(i)
Malignant disease of the lower respiratorytract.
(ii)
Pulmonary asbestosis
(iii)
Pulmonary inflammatory conditions due tofungal infection, bacterial infection,
viral infection or parasitic infection.
Sputum is normally collected as early morning deep cough specimens, and is preferably submitted on three consecutive days. It is not advisable to collect sputum specimen after a recent bronchoscopy has been done.
The result will be invalidated by the presence of numerous inflammatory cells which could obscure some underlying pathology. As much as possible, sputum should be sent to the laboratory promptly and smears made as soon as possible. However, sputum specimens are well preserved when refrigerated at 4°C.
Preparation of smears
Sputum must be processed in a biological safety cabinet. Purulent or blood stained particles are selected from the sputum with a microbiological wire loop and used to make thin smears, at least two smears from each specimen.
Bronchial
washings are usually submitted in sterile containers. They are centrifuged
without delay and smears made from the sediment. They can also be spun at 150
rpm for 10 minutes in a cytocentrifuge directly onto a clean pre-labelled
microscope slides and fixed immediately,
Fixation Fixation should be carried out while
the smears are still wet. Many workers prefer to use 3% acetic acid in 95%
alcohol.
Pleural Fluid and Ascetic Fluid
These are
serous fluids that normally lubricate the walls of pleural and peritoneal
cavities. They increase in volume and contain cells under certain pathological
conditions. Cytological examination of these fluids may reveal malignant cells which
may arise from tumours of the surrounding mesothelium or they may be metastatic
deposits.
Collection and preparation of smears
By means of a needle or canula with an attached syringe, the specimens are aspirated from the pleural or peritoneal cavities. The aspirated material is transferred into a sterile container and sent to the laboratory. The specimens are centrifuged at 300 rpm for 10 minutes or cytospun at 1500 rpm for 10 minutes, and thin smears made, at least two smears from each specimen. Any clots that are formed are fixed and processed histologically,
Fixation Fixation, as usual, should be carried
out promptly and while still wet. However, if the choice of stain is
Romanowsky, then the smear should be air dried and then fixed with methanol
before commencing the staining procedure.
Gastric Brushing or Lavage
These types
of specimens are very useful in the diagnosis of squamous cell carcinoma of the
oesophagus or adenocarcinoma of the stomach. The collection of specimen is by
the insertion of a small brush into the oesophagus or into the stomach under
endoscopic guidance for by X-ray guidance.
The brush is rotated at the particular spot of interest; the material is then smeared on a clean pre-labelled microscope slide and fixed immediately. If specimen of washing or lavage is obtained, then this is treated as the pleural or peritoneal fluids.
The brush is rotated at the particular spot of interest; the material is then smeared on a clean pre-labelled microscope slide and fixed immediately. If specimen of washing or lavage is obtained, then this is treated as the pleural or peritoneal fluids.
Urine
Urine
cytology is of great value in the diagnosis of urethral tumours, urinary
bladder carcinoma, car cinoma of the kidney and carcinoma of the prostate in
males. Normal urine contains few or no cells; but under certain pathological
conditions, the urine contains many abnormal cells. Early morning specimens of
urine are preferred because they give larger concentration of cells due to
relatively long residence in the bladder.
Fixation Urine tends to wash off slides
during fixation and staining due to the low protein content. This difficulty
may be overcome by
(i)
Centrifuging the sample and making smears with the sediment on albuminised
slides;
(ii) The
urine sediment is mixed with some drops of egg albumin and then smeared
or
(iii)
Celloidinising of the slides after fixation. An initial fixation or
preservation can be done on urine sample that may not get to the laboratory in
good time. This is by adding 50% alcohol to the specimen in equal
proportion.
Staining For non-Gynae-cytology, the
Papanicoloau stain is equally as useful as in the Gynae-cytology but many
workers prefer to use it as a confirmatory procedure. The common staining
methods are:
The Romanowsky staining method
Following fixation in ether-alcohol,
smears may be stained by any of the Romanowsky's stains. Alternatively, a fluid
specimen may be smeared and air dried and then fixed with methanol for five
minutes before applying the Romanowsky stain. For technique of Romanowsky
staining, please refer to the Haematology Section
The staining
is satisfactory for cells in fluids and effusions.
The most popular Romanowsky stains are the Giemsa and the Leishman stains. Methylene blue This single-stain, rapid and simple method is useful for the screening of fresh specimens, especially sputum, for malignant cells. The preparation is not permanent and should be examined immediately.
The most popular Romanowsky stains are the Giemsa and the Leishman stains. Methylene blue This single-stain, rapid and simple method is useful for the screening of fresh specimens, especially sputum, for malignant cells. The preparation is not permanent and should be examined immediately.
Staining solution
Methylene
blue 1g
Distilled
water100 ml
Procedure
1. Place a
small amount of fresh purulent sputum, or two drops of centrifuged deposit of
the body fluid on a clean microscope slide.
2. Add one
drop of the stain to the specimen on the slide and mix the two together
thoroughly.
3. Cover the
mixture with a clean cover slip and spread by gentle pressure
4. Examine
immediately.
Results Nuclei: shades of blue.
Any
suspicious cell is confirmed using other more specific staining techniques.
Acridine Orange Fluorescence Method
This method
is based on the principle that the fluorochrome dye, acridine orange, which has
an af finity for nucleic acids, can emit visible light when excited by an ultra
violet or blue light, usually of 350-400 nm Wavelength. At pH 6.0, this dye
will demonstrate DNA green or greenish-yellow and RNA orange-red with
fluorescence microscopy.
Malignant cells have a large amount of RNA in their cytoplasm and so they are readily seen by their orange or red fluorescence under low power magnification. This method permits quick scanning of smear preparation. Positive or doubtful cases are usually confirmed by Papanicoloau stain.
Malignant cells have a large amount of RNA in their cytoplasm and so they are readily seen by their orange or red fluorescence under low power magnification. This method permits quick scanning of smear preparation. Positive or doubtful cases are usually confirmed by Papanicoloau stain.
Solutions
1. 0.067M Potassium dihydrogen orthophosphate:
Dissolve 9.072g of potassium dihydrogen phosphate (KH,PO) in 1000 ml distilled
water.
2. 0.067 M
Disodium hydrogen orthophosphate: Dissolve 9.465g of disodium hydrogen
ortho-phosphate, anhydrous (Na HPO) in 1000 ml distilled water.
3. Phosphate
buffer (pH 6.0): 87.8 ml of solution1 are mixed with 12.2ml of solution
2
4. Acridine
orange stock solution: Acridine orange (CI No.46005) 0.1 g Distilled water 100
ml Store in a dark bottle at 4 °C.
5. Acridine
orange staining solution: Acridine orange stock solution 10 ml Phosphate buffer
(pH 6.0) 90 ml
6. 0.1 M
Calcium chloride differentiator: Calcium chloride (CaCl2) 11.099 g
Distilled
water 1000 ml.
Procedure
1. Fix smear
in ether-alcohol mixture for 15 minutes.
2. Pass
through descending grades of alcohol (80, 70 and 50%) to distilled water.
3. Rinse
briefly in 1% acetic acid and wash in two changes of distilled water for one minute
each
4. Stain in
acridine orange staining solution for three minutes.
5. Wash in
phosphate buffer for one minute.
6.
Differentiate with 0.1M calcium chloride until the nuclei are clearly outlined
about one minute).
7. Wash
thoroughly with phosphate buffer solution.
8. Mount
with a cover slip using phosphate buffer as the mountant.
9. Examine
by fluorescence microscopy.
Results
RNA fluorescence :
|
red
|
DNA fluorescence:
|
green
|
Note
Certain
normal cells and micro-organisms also show varying degrees of orange-red
fluorescence. Therefore experience is required to be able to identify cells and
cellular morphology.
Haematoxylin and Eosin Method
Following
fixation, it is possible to bring smears to water through descending grades of
alcohol and then stain with haematoxylin and eosin as for sections. The H and E
gives good result but lacks the transparency of cytoplasm that is seen in
Papanicoloau technique.
FINE NEEDLE ASPIRATION CYTOLOGY (FNAC)
Before the
advent of this procedure about two decades ago, the needle cone biopsy was the
method employed to
collect material from lesions in the organs that do not normally exfoliate
cells. The fine needle aspiration technique has almost completely superseded
the more traumaic method of cone biopsy.
For
aspiration of specimens, the FNAC basically requires a sterile syringe and
needle, the length of the needle depending on the location of the organ to be
sampled. The needle thickness is usually in the range of 0.5-0.9 mm. A special
handle can be attached to the syringe to allow single hand grip, freeing the
other hand for palpation and fixation of the mass if it is mobile (Fig. 16.4)
To perform
the aspiration, first of all, the skin is cleaned with a suitable antiseptic.
The mass is then immobilised with one hand. Without the piston of the syringe
being retracted, the needle is inserted into the mass. The cells are then
aspirated into the needle. The needle can be inserted from different angles if
the material from one site is too scanty. Release the piston before withdrawing
the needle. This is to equilibrate the pressure in order to prevent the
material being drawn into the barrel of the syringe.
The
collected specimen is expressed on to a clean pre-labelled slide (or slides)
and spread evenly. Fixation is done immediately and stained with Papanicoloau
or H and E stains.Any tissue fragments seen in the aspirate are fixed,
processed and stained histologically. Lesions that cannot be localised by touch
(non-palpable) are visualised by means of ultrasonography or fluoroscopy. The ultrasonography
is for abdominal aspiration while fluoroscopy is used for bone and thoracic
lesions.
FNAC is very
valuable for preliminary diagnosis of carcinomas as well as inflammatory
conditions. It is rapid and fairly inexpensive. But experience is required to
correctly interpret staining result. FNAC is particularly useful in dealing
with suspected masses or lesions on the skin, lymph node, breast, thyroid,
liver, kidney, lung and bone.











If you have any queries related medical laboratory science & you are looking for any topic which you have have not found here.. you can comment below... and feedback us if you like over work & Theory
.
Thanks for coming here..