METHODS USED FOR CHEMICAL ASSAYS
 |
| METHODS USED FOR CHEMICAL ASSAYS |
Spectroscopy
A part of white light passing through certain coloured
solutions is absorbed in relatively narrow areas of the spectrum, giving dark
regions known as absorption bands. The position of these bands can be used to
identify the coloured material in solution. A vision spectroscope is used to
observe these absorption bands.
Flame Emission Spectroscopy
This is also referred to as flame photometry or flame
spectrometry. When some elements are heated directly in a flame, their atoms
become excited and emit energy at a wavelength characteristic for the element.
The intensity of the flame colour is directly proportional to the concentration
of atoms in the sample.
- Flame photometry is commonly used for elements such as sodium (yellow flame), potassium (red-violet) and lithium (red). The flame intensity can be measured in a flame photometer using appropriate filters. The flame photometry is described in previous of this blog.
Potentiometry
Potentiometry compares the potential difference between two
electrodes immersed in a solution un
der zero current conditions. By using a reference electrode
of known potential, the charge on the other indicator electrode can be
determined.
- The most commonly used reference electrodes are the saturated calomel electrode and the silver-silver chloride electrode. Indicator electrodes can be a platinum wire, a carbon rod or a thin stream of mercury.
- In clinical chemistry potentiometry is most often used for the measurement of pH. The pH meter is described in Section I of this book.
calorimetry
Coulometry measures the current passing between two
electrodes. When constant current is applied to generate a titrating agent, the
time required to titrate the sample is related to the amount of analyte in the
sample.
- The most common clinical application of calorimetry is in the determination of chloride ions in serum and other body fluids. If a constant current is applied across silver electrodes, it liberates silver ions into the solution at a constant rate.
- When all the chloride ions in solution have combined with the silver ions, the excess silver ions in solution activate a relay to stop the titration. The length of time of this titration is directly proportional to the concentration of chloride ions.
lon Selective Electrodes (ISE)
If a metal rod is placed in one of its salts, it aquires an
electric potential. If two different metals are immersed separately into their
own salts, the difference in their potentials can be measured. By using a
standard electrode, the potential of all other electrodes can be compared.
- An ion selective membrane is required to separate the solution of unknown activity from the detecting system.
- If the unknown solution has different concentration of the particular ion from that of the solution filling the electrode, the ions migrate towards the lower concentration. This brings about a change in potential across the membrane.
- The process goes on till an equilibrium is established across the membrane. The number of ions transported across the membrane is related to the original concentration of the ion in the unknown solution.
- Ion selective electrodes (ISE) are used to measure dissolved gases such as Pco, or serum electrolytes such as Na and K.
Ultraviolet spectrophotometry
The wavelength used for spectrophotometry depends on the
wavelength at which there is significant absorption by the substance to be
measured. If the wavelength of the light absorbed is in the ultraviolet region
of the spectrum which is non-visible (200-400 nm), the analysis is called
ultraviolet spectrophotometry.
Turbidimetry and Nephelometry
When light strikes a particle in a liquid, the light can
either be absorbed, transmitted, reflected orscattered. Light scattering can be
measured by two methods, turbidimetry and nephelometry.
Turbidimetry
Turbidity causes a reduction in the intensity of the
incident light as it passes through a solution of particles. Turbidimetry is
the measurement of this loss in intensity because of scattering, absorption or
reflectance of the incident light.
- Turbidity is measured at 180° from the incident beam in the same manner as absorbance is measured in a spectrophotometer. Most colorimeters and spectrophotometers can measure turbidity with good precision and accuracy.
Nephelometry
Nephelometry is the detection or measurement of light
scattered or reflected toward a detector which is not in the direct path, i.e.
at 180°, of the incident light. The instrument used for nephelometry is called
nephelometer.
- Most nephelometers measure scattered light at 90° to the incident light. Figure 1.7 shows a schematic diagram of the working principle of turbidimetric and nephelometric measurement.
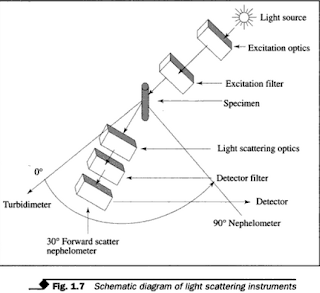 |
Schematic diagram of light scattering instruments
|
Light scattering instruments are very useful in immunoassays
of specific serum proteins, immunoglobulins, coagulation factors and therapeutic
drugs.
Osmometry
Osmometry is the measurement of osmolality of a solution.
The osmolality of a solution depends on the number of particles in solution.
The size, charge or mass of the molecule does not affect the measurement.
- One osmole of a substance is equal to the gram molecular weight divided by the number of particles or ions into which the substance dissociates in solution.
For example, NaCl dissociates into two ions (Na+,
ci-) in solution
One osmole can be measured as the mass of the solute which
when dissolved in one kg of pure water produces an osmotic pressure of 22.4
atmospheres at normal temperature and pressure (NTP): or as the mass of the
solute which when dissolved in one kg water depresses the freezing point by
1.86°C.
Osmolality is determined by measuring the depression in
freezing point.
- An osmometer consists of a cooling bath to freeze the specimen, and a thermistor. A thermistor is a device whose electrical resistance increases as the temperature decreases. The resistance of the thermistor is so calibrated that it is directly related to the temperature being measured. The digital display on the instrument directly gives the osmolality of the solution.
- The osmometer is most often used to measure the osmolality of urine. It differs from specific gravity, which is the 'mass' concentration of solutes while osmolality is the molecular' concentration. Specific gravity is the ratio of the mass of a solution compared with mass of an equal volume of water.
- It does not measure the exact number of solute particles, but depends on both the nature and number of particles. Osmolality, on the other hand, depends only on the number of particles. However, there is a good correlation between osmolality and specific gravity under most circumstances.
Fluorometry
Fluorometry is the measurement of fluorescence. When a
compound (fluorescent light energy from one wavelength, an electron is raised
to higher energy level. Part of the absorbed energy is used up.
- When the electron falls back to the original state, the excess energy is emitted as light in another wavelength; and the substance is said to fluoresce. The emitted light has a lower energy level (higher wavelength) than the absorbed light.
- For example, some fluorescent substances absorb light energy from the invisible ultraviolet rays (wavelength 340 nm) and emit light in the visible spectrum (400-700 nm). Fluorescein isothiocyanate (FIT) absorbs UV light and fluoresces green light. The instrument used to measure fluorescence are fluorometers or spectrophotometers.
The basic components of these
instruments are-
(i) the excitation source
(ii) the excitation monochromator
(iii) the sample cell (cuvette)
(iv) the emission monochromator and
(v) the
detector.
- Figure 1.8 shows a schematic diagram of a spectrophotometer. The fluorometer uses a glass filter or interference filter to produce monochromatic light for excitation whereas the spectrophotometer uses a diffraction grating or a prism.
- The energy source for fluorometry is generally a mercury arc lamp or a xenon lamp. The emission monochromator and the detector are at right angles to the incident light beam. This prevents the high energy incident light from reaching the detector.
- Fluorometry is an extremely sensitive technique and is very widely used for immunochemical measurements for the analysis of drugs, peptides, hormones and other compounds present in very low concentrations.
 |
| working principle of fluorometer |
Immunochemical Assays
Immunochemical assays make use of the antigenantibody
reactions. Antigen is a foreign substance, which when introduced in the body
stimulates the production of an antibody and reacts specifically with it.
Antigen, antibody and their reactions are discussed in the Basic Immunology
section of this book.
- Because of their high sensitivity and specificity, immunological reagents and methods are widely used in clinical chemistry. Antigenic properties of some drugs and hormones are used to raise specific antibodies in experimental animals.
- These antibodies are used as specific reagents for the detection and quantitation of drugs or hormones in patients' specimens. Serum immunoelectrophoresis is useful for the detection of abnormal fractions in the serum, which combines immunoassay with electrophoresis.
RADIOACTIVITY
An atom of an element consists of a small, central nucleus
of positive charge surrounded by orbital electrons, each carrying a negative
charge. The nucleus is composed of protons (with single positive charge) and
neutrons (with no charge). The atomic number of an element is the number of
protons in the nucleus, where as the mass number is the total number of protons
and neutrons. A nucleide is an atomic species (element) with given atomic
number and mass number.
for more click here







If you have any queries related medical laboratory science & you are looking for any topic which you have have not found here.. you can comment below... and feedback us if you like over work & Theory
.
Thanks for coming here..